Respiratory Syncytial Virus (RSV) Vaccine – Arexvy
HKD $2,800
- Registered by the U.S FDA, CDC, and Hong Kong Department of Health
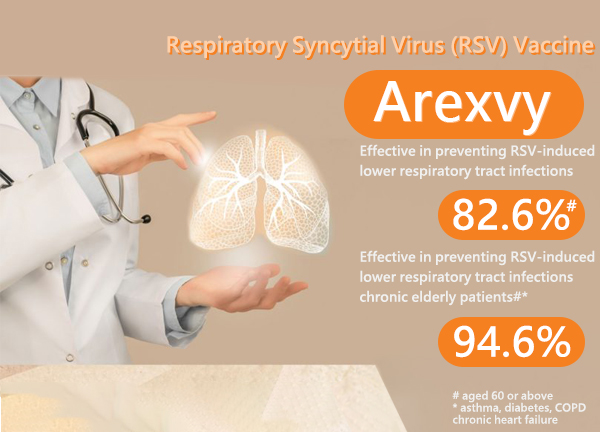
- Highly effective in preventing RSV-induced lower respiratory tract infections, with a remarkable efficacy of 82.6%
- Individuals aged 60 and above (especially those with chronic diseases) are advised to consult their doctors to discuss vaccination strategies.
Introduction
FDA-approved vaccine to prevent lung and lower airway infection from respiratory syncytial virus (RSV) in people 60 years and older.
- Available for people aged 60 and older
- Can be given simultaneously as a quadrivalent flu vaccine
- Only a single-dose shot is required
In a clinical study, AREXVY was
- Over 82% effective in preventing lung and lower airway infection from RSV in people aged 60 years and older;
- Over 94% effective in preventing lung and lower airway infection from RSV in people aged 60 years and older with asthma, diabetes, chronic obstructive pulmonary disease (COPD), chronic heart failure, advanced liver or kidney disease, or any chronic respiratory or pulmonary disease.
Manufacturer
GlaxoSmithKline, GSK
Details
What is RSV infection?
RSV infection is caused by the respiratory syncytial virus (RSV). RSV is a common virus that primarily affects the respiratory system, particularly the lungs and airways. It is a leading cause of respiratory tract infections in infants, young children, and older adults. Common symptoms include coughing, sneezing, runny nose, sore throat and fever. In most cases, RSV infection causes mild, cold-like symptoms. However, in some individuals, particularly those with weakened immune systems or underlying health conditions, RSV infection can lead to more severe complications, such as bronchiolitis or pneumonia. Severe patients need hospitalisation for treatment.
Who can get the RSV vaccine, AREVXY?
AREVXY is available for people aged 60 and older.
How is AREVXY given?
AREXVY is a single-dose injection given in the upper arm.
What are the most common side effects of AREXVY?
The most common side effects of AREXVY are:
- Injection site pain
- Tiredness
- Muscle pain
- Headache
- Joint pain
Service Location
Virtus Medical Tower – 17/F, Virtus Medical Tower, 122 Queen’s Road Central, Central, Hong Kong
Virtus Medical Centre – 11/F, H Zentre, 15 Middle Road, Tsim Sha Tsui, Kowloon
Terms & Conditions
- Advanced booking is required for the vaccination service.
- The appointment date and time have yet to be confirmed. You will receive a call from our customer care team within 2 business days to confirm your booking.
- Service(s) must be booked and used within 90 days from the date of purchase.
- The fee includes the doctor’s consultation charge on the first visit.
- Health Care Vouchers are not applicable for online purchases. If you wish to use Health Care Vouchers, please call 38936311 (Central) or 36912000 (Tsim Sha Tsui) to make an appointment.
- Other treatment, investigation or drug costs are not included in this service.
- This service is non-refundable and non-transferable.
- This service is only available for rescheduling once within 30 days from the date of purchase.
- If you are unable to attend due to medical conditions, please provide a medical certificate.
- Service(s) must be booked and used within 90 days from the date of purchase.
- Virtus Medical Group reserves the right to amend the terms and conditions of the above service without prior notice. In case of any dispute, the decision of Virtus Medical Group shall be final.
You may also like…
Adult Vaccines
Related products
Adult Vaccines
Adult Vaccines
Adult Vaccines